26 February 2014
Eye on the Future:
How can modern scientific knowledge help to prevent blindess?
Professor William Ayliffe
Ladies and gentlemen, welcome to Gresham College. My task this evening is to talk to you about the future developments that are going to happen in medicine, particularly in eye care. Some of the things that I will be talking to you about are already in the clinics, many of them are in translational research, and only some of those will actually become a reality. In fact, many of the things that we see in the clinic will possibly die out as other treatments are shown to be more effective in the future. It is a very exciting area.
I am going to begin though with the old times, and we are going to move forward to explain how gene therapy came about.
Originally, we had problems with anti-infectious agents because, until the dawn of antibiotics, there was really only ways of killing bacteria that could also damage the person or the animal you were treating as well. But as soon as antibiotics were developed, antibiotic resistance became a problem, and became a problem very quickly, and spread very quickly, through mobile genetic elements, and there is a number of different ways that this could occur. These were studied in detail in the 1950s and ‘60s, and set the scene to allow scientists to think about using mobile genetic elements to replace defective genes in humans and animals.
This is not the whole story and there have been main problems and pitfalls on the way. It is rather more difficult to do than you will get the impression from the simplified version I am going to give you tonight, but nevertheless, for previously untreatable eye diseases that would inevitably lead to blindness, there is now hope and some very encouraging results coming from clinical trials.
Finally, I will very briefly mention some of the other developments that are going on with prosthetics, optogenetics, and stem-cell transplants.
The story begins with Gregor Mendel, who was a monk in this institution. He was discouraged from doing his experiments on hybridisation with mice by his bishop. He commented, wryly, that the bishop did not understand that plants also have sex, and went on to study the variations in the pea plant. Luckily for him, he chose seven variations that occur independently of each other, but he worked very hard and made thousands and thousands of generations of these peas, and what he discovered was something that was very, very important. What he discovered was that the primary parent generation, if they were purebred, led to the first generation, the F1, having just one characteristic of either the mother or the father, but the next generation, from self-pollination, the recessive feature reappeared, and this reappeared consistently in a three to one ratio, and the numbers he had were really large. So, the 700-odd purple flowers, 224 white flowers, and this ratio was almost exactly three to one – these are large numbers. They were criticised by Fisher in the 1930s and ‘40s, but subsequently, it has been found that, taking into account how the experiments were done, they represent true results and that Mendel did not actually invent his findings.
So, he tried to find an applicable mathematical law. He had trained in Mathematics before he came to this part of the world to do these studies in the gardens, and he came up with the ratio that you can see here: A/A + A/a + a/A = A + 2Aa + a. Now, some of you with horrible memories of O Level Maths will recognise that as a quadratic equation. What he had found was a fundamental mathematical law of God, which was one of the things that had actually driven religious people in studying science for over 1500 years. Furthermore, it did not just apply to the flower colour. It applied to the seed colour. It applied to the colour of the pods. Every single time he did these experiments, with large numbers, he was finding that the second generation, the recessive phenotype reappeared at a three to one ratio.
Later, this was going to be interpreted by Correns and others as having two components were being inherited for each of the phenotypes, and one of these was a dominant and one of them was a recessive. This is not what Mendel did. Mendel did extraordinary work and presented, through his co-workers, very good results on hybridisation, which was his field. He was not ignored. In fact, his work was presented in London and was very well received. Now, do remember that Mendel had no concept of what a gene was. By the way, nor did Darwin. He was working on the possibility of there being a gemmule, but he did not really have any concept of what genetics was about because that had not been invented yet.
Now, when Correns actually made the postulation that in fact there were units of hereditary being passed on through the generations, the next questions was: what were these units? The commonest thing to believe, in those days, was that in fact they were going to be proteins, because proteins had been recently studied and analysed and it seemed to be a very reasonable assumption.
Also, the site of the location. Well, Robert Brown, who had sailed with Flinders to Australia, and remember this as one of those many anniversaries that comes up I think next year, was the first person to document the nucleus in cells, actually in plant cells.
Miescher discovered a very important thing, he discovered the nuclein which was staining in white blood cells, and that is what Walther Flemming put the aniline dyes into to make these beautiful drawings here that demonstrate these wispy, dark-staining structures within the nucleus of the cell. Many other experiments were done which showed that chromosomes were needed to pass on the information.
Sir Archibald Garrod discovered that Alkaptonuria obeyed a recessive trait – this was the first metabolic disease that had had its genetics worked out.
We are moving now, very quickly, from peas and pea colour to animals and real diseases, and this is an extraordinary moment in the history of science.
William Bateson – here is the cartoon of him holding his ducks. He created the term “genetics”, meaning “to give birth”. The word “gen” had been earlier used by the Danish, with a similar origin.
Eventually, genes were shown to be on chromosomes, and then a genetic map of the chromosome was made, and then DNA was found in chromosomes in the 1930s, and the RNA, which is the ribonucleic acid, was not in the nucleus – that was found to be in the cytoplasm. And then a number of other experiments went on, which were going to lead to the important discoveries enabling us to consider gene therapy.
So, we know that the genetic material is DNA, deoxyribonucleic acid, and the genes are located in the DNA, which is tightly, tightly wrapped up to form the beautiful chromosome structures that Walther Flemming had discovered with his aniline stains in the previous century.
Then, Watson and Crick, using Rosalind Franklin’s data – and this is her x-ray of the DNA crystallised molecule – worked out that this represented a helix, and after some experimentation - and this is their original model, made out of test-tube clamps and pieces of glass, and a reconstruction of this can be seen in the Science Museum today – they came to the astonishing conclusion that it was not just a helix, it was a double-helix, an intertwined helix. This was the letter to his son which he is showing here with his excitement at what had been discovered.
Now, how much is present in a gene? Well, a human genome has about three billion of these base-pairs. Bacteria such as e-coli has less, would have 4.6 million. The interesting thing is that, of all these three billion base-pairs, more than 99% of them are identical between everybody in this room and between everybody throughout human existence. But I look in this room and I see tremendous variation in phenotype and that means what people look like. I see variation in hair colour, mainly further towards the back than the front, I admit, and colour of eyes.
Now, this 1% of the differences is responsible for all of this, and some areas of the genome are variable, so we can have alternative variations of the gene. If they occur reasonably frequently, these alleles are called polymorphisms, and they have got to occur in 1% of the population to become a polymorphism. There are very few simple Mendelian traits when we look at human physiognomy. For example, the eye colour is related to many genes. But, for simplicity, we could say blue and brown eyes, for example, and the difference between the blue and the brown has a genetic basis, and the differences between those are allelic. So, you can have people who have brown alleles and you can have people who have blue alleles, and in fact, the mutation that led to blue eyes occurred relatively recently in human history, in the time after the Ice Age, and was a sexual selection so that blue-eyed males would select blue-eyed females to ensure that their children were their own because, if they ended up with a brown-eyed one, they could be pretty sure that they were not the parent of that child. That is believed why this rare allele, that otherwise has no use whatsoever, was so highly-selected and becomes more and more common as we spread throughout Europe, going towards the North and West. It is the inability to make a protein – it is a recessive gene.
The most famous example which was in our Biology textbooks, and then we went to medical school and were told, oh no, that was all wrong, and now I have just been reading up some stuff for this lecture and found that actually it is all right again, is the one of the moth. The natural state of this moth is the peppered state, the peppered moth, and it is very good at camouflage. It is very difficult, even for those of us without macular degeneration in this audience, to actually see that moth on the normal tree, but in the Industrial Revolution, trees did not look like that. Trees looked black and brown, and so did buildings, covered with soot, so you would show up very, very easily if you were a white moth, and you would become food, bird food, and that is what happened. So, there was a rare allele in the moth population that allowed them to make melanin. Those that made the melanin were much more difficult to spot on the darker trees and therefore lived to survive and to breed, and in fact, by the 1890s, the melanotic moth was the commonest form of the peppered moth in Manchester. This is a sort of Darwinism in action.
How is this code read? Well, we know that there is a pair of bases, sugar bases, which are holding the DNA coil together, and these can only pair in a certain way. What happens is, the DNA is read by an enzyme that makes a message, called messenger RNA, which is an exact copy. That copy, after some processing, which we will come onto, is taken up to the ribosome, which is a structure made out of proteins and RNA, and then it is translated and made into a peptide. How this is done is very strictly controlled and it only occurs in a certain direction. So, the main things are the copy, that is the transcription, and then the translation of that into a protein.
Before the mRNA leaves the nucleus, it is spliced, as we can see here. It is spliced, so there is a long copy. This bit, which used to be called junk or rubbish DNA is taken out, and the main bits of the gene are spliced together. Now, it turns out this is not exactly junk and some of this is going to be useful for gene regulation, and also what is important is, depending on how you splice it, you can make, from one area of DNA, one gene, several different proteins, and this is what is done, for example, with antibody molecules or the receptors on immune T-cells. So, it is a very important way of generating diversity from a limited number of genes, and it is more important than has previously been recognised. The splicer zone is the protein complex that actually deals with doing this.
I am going to give you a little primer of bacterial biochemistry because you know what is coming next, which is antibiotics. So, we need to understand why bacteria are different. They do not have paired chromosomes. They have just one, a great big circular bunch, and all their DNA is here. They synthesize de novo from para-aminobenzoic acid, the dihydrofolate, tetrahydrofolate, which is going to be the precursor to actually build this double helix. They use a slightly different mechanism here of the ribosome, with a 50S and a 30S. It is a slightly different structure, but, again, very similarly to higher animals and humans, the transport RNA carrying the amino acid comes in, is locked in, and then moves down the chain and eventually the protein is formed, and if it is released, this could be a toxin, such as a cholera toxin, or it might be a protein that is needed for the cell, such as the transpeptides that makes the wall.
How the amino acids are added on is step by step, so it grows. So, here is the nascent chain, this is the code, this is the complement that is carried on the transport for the amino acid, and in it locks, so the next amino acid that is going to go on here is going to be red. So, here we go, the next one that comes in is carrying blue, which will be locked in here, and then the pale one will be locked in here, and, gradually, this chain grows and grows and grows, adding on all the different amino acids that there are for making a protein.
In broad terms, there is nothing particularly different from eukaryotic cells – that is cells with nucleuses - in the general terms, but in the detail, there is an awful lot of difference, and it is that detail that enables us to use molecules that can kill these critters and not kill us when we use them.
So, in summary, before we move on to the antibiotics, we have understood now that genetics is the study of hereditary. There are discrete bits of hereditary called genes. The expression of these genes is what gives us the phenotype that makes us a black peppered moth or a peppered pepper moth. How those phenotypes come to populate is determined by natural selection, the same as perhaps blue eyes in Northern Europeans.
When we have variations in genes that do not just change the phenotype, in a non-dangerous way, such as brown or blue eyes, but in a dangerous way, they can cause a disease, such as an inborn error of metabolism, that is very serious, and that can lead to blindness, if it is affecting the eye, or death, if it affects other parts of the body, and correcting those genes was the big challenge. It was considered, not so many years ago, to be an impossible dream, and in fact, after some accidents, the whole project nearly came to a grinding halt.
I am just going to take you back, very briefly, to a pre-antibiotic world, when people routinely died when they caught tuberculosis, where a cricketer, a famous cricketer, who has scored many runs in Melbourne on the Ashes tour, could slip on a dance floor and die from septicaemia from a grazed elbow, where the son of the President of the United States could get a trivial injury playing tennis and get it infected and be dead within three days. There was nothing anybody could do about this.
I want you to consider the future, where, if we end up with multi-resistant bacteria, for which there are no antibiotics, we are back in this world again. That future is with us now and has already happened, and I will go through and I will show you what I mean by that.
Furthermore, unless we do something about this, it will not be infrequent frightening events, it will become the norm, and there are very few people alive in this room now who can remember when epidemics of infectious disease would take out whole communities. Dozens of children would suddenly not appear back at school.
It affected people such as soldiers. In the First World War, I mentioned in my last lecture, that in fact the second commonest cause of soldiers not appearing for duty was venereal disease, mainly gonorrhoea, and the British Army of course, in its traditional way, said these Christian soldiers cannot possibly get venereal disease, so they did nothing about it. But a much higher incidence in the New Zealand troops led to something had to be done about it. Now, there were no antibiotics. The only thing you could do was to make licenced brothels, such as Maxine’s Bar, where soldiers displaying obvious infectivity and the girls working in those bars displaying obvious infectivity were barred, and this reduced the rate very much. This was the German mechanism of dealing with it, and that is why they had a very low rate of venereal disease.
Then this person, Ettie Rout, whose name is forbidden to be mentioned in New Zealand, on pain of a £100 fine. She is actually a hero. She was given honours by the French after the First World War for setting up these bars and providing her prophylactic kit.
Between the First and the Second World War, experimenting on bacteria, we had realised that certain chemicals could dye bacteria. Now, if they could dye bacteria, as Christian Gram had shown, could they kill bacteria? And Domagk, who had fought at Ypres, found this Prontosil. Now, the great thing about Prontosil was that it did kill bacteria, but it dyed the patient red.
He accepted the Nobel Prize, but was arrested by the Gestapo, briefly, because Carl von Ossietzky, who had been the editor of the anti-Fascist Die Weltbuhne, was currently in a concentration camp for revealing the German rearmament to the world, and he had been given a Nobel Prize. So, the Nazi Party were not pleased about Nobel Prizes and forbad anybody to accept one. Unfortunately, he was to die in the concentration camp of tuberculosis.
Prontosil was actually not a unique drug. It had been discovered previously as a dye, so unfortunately, they could not patent it and make a fortune. But, very quickly, May & Baker, over here in England, did, and they made sulfanilimide, which did not dye the skin red and was far more tolerable, and a lot of me-to drugs came onto the market, some of them with tragic effects, when 100 children died of ethylene glycol poisoning with the elixir of sulfanilimide in 1937. But these drugs changed the world. Suddenly, women did not die in childhood of sepsis. Children with sore throats did not die. People with infected wounds no longer died. This was a major advance and it was the first of the modern antibiotics.
In fact, interestingly, this German invention saved Churchill’s life. When he was coming back from the Tehran Conference, he fell ill with pneumonia in North Africa. He was given sulfapyridine, May & Baker, which had now been licenced to the Americans and was available in vast quantities, but was put on a strict diet that allowed him only to drink weak whiskey and soda and not to smoke at all – no cigars. He complained bitterly to his nurse. He said, “Pray remember that a man cannot live by M&B alone,” which is the May & Baker sulfapyridine. That he was treated with the good old British penicillin is, of course, a myth.
It is also a myth that penicillin was used in these situations – it was not. What were used was crystalline sulfanilamide and sulfadiazine tablets. Penicillin was far too precious to be used for war wounds. It was needed to get 11,000 soldiers a week back into the fighting line who were getting gonorrhoea in Alexandria and various other places, and the treatment, the old treatment, was taking several weeks to a month, and, for syphilis, could take six months. So, penicillin was reserved for treating those people.
We know that Alexander Fleming discovered it when he noticed that a mould inhibited the growth of bacteria in a petri dish. His was not as neat as this, and in fact, that is a photograph, a famous photograph, of that original petri dish in the laboratory in Praed Street.
Now, Ernst Chain was important because this penicillin mould, you know, there is not enough penicillin here to treat a mouse for a day. You need to make tons of this stuff. So, they started manufacturing it in biscuit tins and collecting all the mould juice, and eventually, they had enough. And a policeman who had cut himself on a rose thorn in his garden was dying and had already had half his face removed surgically, and his eye, to try and control the rip-roaring streptococcal infection, he was given all the penicillin that was available, and came out of his coma and was able to meet his family who came to see him. The next day, they collected his urine, re-purified the penicillin and put it back into him, and he survived another day. The third day, there was not enough penicillin left and he died.
This lesson taught the antibiotic producers that they needed mass production. So, where do you go for mass production? The home of mass production – America! So, they went off to America and went to the Department of Agriculture, where they were used to manufacturing vast amounts of fermentation products. Unfortunately, things went wrong. Initially, they went right. They found that, if they used a corn-steep liquor, it improved the amount of penicillin produced by the cultures. They also found that there were other varieties of penicillin, ironically enough, one in a Peoria fruit market just down the road, when they scraped it off a melon, produced ten times the amount of penicillin than the old St Mary’s culture did. So, a lot of work was done. A lot of patents were filed, which was what led to some bad taste between the English researchers who had given their work for free. Then penicillin eventually comes onto the market.
In 1943, the first shipment arrives in North Africa, and a big fight. Now Colonel Edward D Churchill, no relation recently but maybe distantly, said that this is going to be used for treating war wounds. It was considered, by Chain and various other peoples, an anathema to use this for what it needed to be used for, which was gonorrhoea. So, the chief surgical consultant, the theatre surgeon, applies directly to Churchill, and Churchill makes the famous order, that it can be used to its best military advantage, and if that means those wounded in brothels, so be it. So, that is why penicillin was used to treat this disease.
Now, it was not all just – the feminists say this was terrible, everyone is blaming the women for this disease. Well, look at this poster: “Syphilis – all these men have it! Women, stay away from dancehalls!”
Eventually, there was enough production that did enable them to start to use penicillin for battle injuries later on in 1944, and, by that time, they had reduced VD by 30-fold. It was an amazing advance, but there were still 600 incapacitated every single day, and when you have got a shortage of military personnel, this is a significant effect that can make the difference between winning battles and losing battles, so this is why penicillin was very, very important and probably made a big difference in that North African campaign – but not from treating battlefield injuries.
Of course, we did not invent antibiotics. They were invented by microorganisms 3.5 billion years ago, for a number of different reasons to get a competitive advantage in the hot sweltery, slippery pool that you were evolving in, and very quickly, as soon as higher animals developed, such as leaf-cutter ants… This is an extraordinary picture. This is not a lot of leaves on the floor. This is a lot of leaf-cutter ants carrying the leaves back to their den, and in fact, they are the major grazers of the neo-tropics, the leaf-cutter ants. What is interesting is they garden the leaves and they grow a fungus on it. Now, the problem with the fungus is the fungus has a parasite that can kill it. So, what they do is they make another culture, with actinomycetes, that produces many antibiotics, and this stops the bad microorganisms getting in and destroying their beautiful garden and fermenting leaf crop that they need for survival. It is a very ancient system, and in fact when the queen flies on her flight to set up a new den, she will take a bit of the fungus with her and she will also take a bit of the bacteria to produce the antibiotic to ensure that fungus will survive. So, when you are busily there spraying your tomato plants and so on, thinking, hey, this is really good, is it not wonderful how advanced we are, you realise that, 50 million years ago, leaf-cutter ants had exactly the same mechanism of looking after their gardens.
Very, very quickly, people realised that, if one mould, penicillium, can produce an antibiotic, there must be some others. So, in Waksman’s laboratory, which was an agricultural research unit, they went on a search and they searched everywhere. They went into the soil outside. They went and scraped under fingernails. They went and had a look under stones. They sent missionaries off abroad, who sent back funguses, and actually, from one of those, in 1953, vancomycin was eventually cultured, and this is one of the drugs that we use for multiple resistant staphylococci to this day.
The most important drug was streptomycin because this changed the world because it allowed the first antibiotic treatment for TB. There was a great fight because Schatz, who had done a lot of the groundwork and in fact had actually isolated some of the original antibiotics, was not mentioned in the Nobel Prize award, and he was furious. Secondly, Waksman had done some patents and he had not used the money he was getting for the patents entirely for noble purposes, and a stinking fight fell out. Waksman said it is because he was jealous, he said it is because he is a crook. Eventually, the university did their own research and realised the major contribution that Albert Schatz actually made, although he did die a very unhappy and embittered, but brilliant, scientist, who we have to recognise.
Antibiotics are not easy to find. You have to look at a lot. So, if you take 10,000 actinomycetes species – that is the soil bacteria – and you screen them, 2,500 produce antibiotics. One in one million would produce erythromycin, which is a common penicillin variant for those allergic to penicillin. One in ten million would produce daptomycin. So, we have discovered all the easy antibiotics already. Look how difficult it is to find a novel antibiotic now. This is one of the problems we have because, as we generate resistance, it is more difficult to find the antibiotics.
Now, the mechanism of antibiotics is various. We can inhibit metabolism, and this is how the sulfo drugs work, because they look very similar to PABA, do they not, and so they interfere with this enzyme and you do not make so much DNA, so the bacteria cannot divide and it dies.
We can also inhibit the cell wall, and this is how penicillins work, how vancomycin works, and it sits into a lovely slot here and it stops the cell wall being formed. This, as the bacteria grows, the cell wall bursts and the bacteria dies.
We can actually stop nucleic acid synthesis, with a number of different things to do this. We can use rifampicin, quinolones.
We can stop the cell membrane, with polymyxins and, more recently, with colistin and then we can inhibit the protein synthesis more directly, with chloramphenicol, which you will see as an eye-drop over the counter, clindamycin, tetracyclines and a bunch of aminoglycosides that are used in hospitals but do carry with them some notable toxicity, included that of deafness.
This is how they work: they interfere. They get into this machinery, rather like sticking a glove into the wheels as they are turning round, and it grinds to a halt.
But, look what happened. Even in 1940, Ernst Chain had reported on strains of bacteria that were penicillin-resistant, and this is before penicillin had been released as an antibiotic. So, already, bacteria had already worked out this biological warfare between each other – you make an antibiotic, I am going to make something that is going to stop that working on me, I am going to make another antibiotic, I am going to make something else… This had been going on for billions of years, as life crawled up the greasy slope of evolution.
Now, within very short times, when erythromycin came out, within a year of it being used in Boston, about 20% of strains were resistant, and very quickly 50-60% of strains were resistant and it was not able to be used anymore.
But there are several urgent threats today: clostridium difficile. These are carbapenem-resistant. These are brand new antibiotics and we have got major resistance to these.
There is many, many serious threats: drug-resistant tuberculosis; and there is some that are concerning threats, which are things like vancomycin-resistant stuff, lococcosaurius. Why is that a potentially concerning threat? It is because vancomycin is the major drug that is used to treat MRSA, the multiple-resistant stuff.
How does this antibiotic resistance come about? Well, it may not surprise you to realise, since this is a lecture on genetics, that it is genetic, and there is a number of ways that it can do it. The bacteria can just pick up the resistant genes that are just floating around in their milieu. They can actually have them injected into them by a virus that affects bacteria, called bacteriopharges. They look like little moon-landers, do they not? In it goes, and then it gets incorporated. And of course there is bacterial sex, where they conjugate, and they can pass on the genes to each other this way. This is very handy for passing on big genes, whereas this is passing on little tiny genes which have a limited resistant.
And the big resistant gene in staphylococci is this mecA cassette and it stops beta-lactam antibiotics. Now, beta-lactam antibiotics are things like penicillin that contain this ring.
How do bacteria overcome antibiotics? Well, they can do this in a number of different ways. They can inactivate the drug. They can use an enzyme to destroy the drug. They can modify the drug by adding a group to it that stops it working so it does not fit into its target site. They themselves can alter their target sites, with a genetic allelic variation that prevents the drug working that previously worked very well. They can make another alternative protein that does not bind penicillin. So, transpeptidase binds penicillin very well, stops the cell wall being formed, but it does not bind to the alternative, the penicillin binding protein, which binds penicillin very weakly. This can be used as an alternative transpeptidase to make the cell wall, therefore bypassing – you do not have to destroy the antibiotic. You can be really clever and evolve a different system that does not use the antibiotic, does not need the antibiotic to be there at all, does not care if the antibiotic is there because the antibiotic cannot get at it. And then you can actually get rid of a metabolic pathway, amazingly enough. So, sulfonamide, which we know looks very much like PABA, and bacteria mainly need PABA to generate this pathway for the folate, there are some that just do not use PABA anymore, and they get this straight from the environment, like we do. We do not make this either, and we need to get it from bacteria in our guts.
So, methicillin resistant staphylococcus A, originally called “meticillin” – “meticillin” was changed to methicillin, and now, the m stands for multi-resistant. Now staph aureus is a very interesting bug – 20% of people in this room will have it living on them, where it causes no harm at all. It is a commensal, usually in the nose, but also in certain skin creases as well. It was found and discovered by Sir Alexander Ogston, and it was called Ogston’s coccus, and he found it in bandages from the surgical wards.
It has many, many virulence factors, this staphylococcus. It can make a number of proteins, it can make a number of toxins that it releases that can cause things, from food poisoning to abscess formation to septicaemia, and when it gets into the body, it can be extraordinarily dangerous. It is also very tough and difficult to get rid of, for a number of reasons, not only just the resistance genes, but it tends to form slimes and lives in slimes. Antibiotics cannot get into these slimes very easily, so these bugs are tough, they are hardy and they are difficult to get rid of, and in fact, if they are living on heart valves, staphylococci, for example, when they infect prostheses, sometimes you are in hospital for months with antibiotics, trying to kill off the bacterial colonies.
This is the beta-lactam ring. The very first resistance was an enzyme, beta-lactamase, that splits the ring, and this is busted penicillin – it is not good any more. So, they invented methicillin, which has got another change in the shape here that does not fit into the beta-lactam ring, but it was not wrong before bacteria, particularly staphylococci, became methicillin resistant. They worked a mechanism, and the mechanism was not to develop another beta-lactamase, the mechanism was to develop that alternative protein, penicillin-binding protein 2 that we mentioned that can carry on making the cell wall.
Now, we have got carbapenems, which have a completely different structure, down here, with these radicals coming off, which, again, makes it more difficult to be destroyed by the bacteria, and it is resistant to most beta-lactamases. But, we have now bacteria resistant to this antibiotic.
What is happening with a lot of these – so, there is different ways we can get the antibiotic resistant genes passed around, as I have mentioned before, but what we want to look at here is this, the mecA cassette. This is the major – there are several variants of this mecA cassette, and they fall into two broad groups really. One group is in hospitals, and hospitals had the original problem with MRSA, but emerging is the community MRSA, and what is interesting about the community hospital is that they tend to have smaller genomes for antibiotic resistance, but they have picked up a very nasty thing from a virus called Panton-Valentine leukocidin, which is a very, very nasty toxin indeed that causes little pimples to turn into abscesses, and abscesses to turn into cavities and to cause significant mutilation and risk of death, particularly amongst things like American footballers.
If you are carrying this, it is not going to do you any harm, unless it gets into your body, but you can come into hospital and you can bring it into hospital with you, and if you do, it is going to have bacterial sex here and, hey, it is going to pick up the multi-resistant gene, and mecA does not just give you resistance to methicillin, there is a whole bunch of other antibiotics it is resistant to. What is more, they are not just having sex with each other, they are having sex with other species of bacteria and passing genes on.
Staphylococci and other cocci bacteria are rather resistant to just picking up genes in the milieu. They do need a mechanism to get it through. But they have found that mechanism. So, having a lot of antibiotic-resistant bacteria in the environment is not a good thing. So, who is hogging all the pills?
Well, this is what antibiotics are used for in our society. They are used as growth promotion in animals. They are used as therapeutic prophylactic in humans – you know, have an antibiotic, it could get worse, take an antibiotic and it will go away. I will come onto that in a moment. They are used in aquaculture – loads of antibiotics are used here to make fish and prawns and various other shellfish that are grown in massive farms, particularly in the Far East, but also just off the coast of Scotland. They are used in culture for sterility and a number of other things such as towelettes. This is madness, absolute madness! The human race, I have come to a firm conclusion over the last three years, is stark raving lunatic and is bent on a self-destructive path.
Now, we keep on blaming the GPs for giving too many antibiotics – that is the general thing, because we like to blame GPs all the time now, do we not? On the new contract, they are not doing nights on-call and everything. But, in fact, they do a very good job, and it is darned difficult to get an antibiotic off a GP, even if you need one.
But farms are using double the amount of antibiotics than humans. Now, you are not allowed to use them as growth promoters anymore – that has been banned in the EU. It is not banned in America, but it is banned in the EU. So, what happens is, Farmer Giles calls in the vet and says, “I think my little porker down here has got the old snuffles,” and he goes, “Yeah, that is not good – that could spread throughout the whole herd. We had better treat all of them.” And, amazingly, this prophylactic use of antibiotics also works as a very nice growth promoter, and when you are talking about the difference between a farm going bust at $1 for a piece of meat and $1.10, then you need to get as much pork out of your animals as you can to keep afloat and keep in business. So, it is not just the farmers’ fault. This complete drive for cheaper and cheaper food production is part of this whole problem. We cannot just look on this and point a finger and say, “Oh, you doctors are very bad! Oh, you vets are very bad! Oh, you farmers are very bad!” We are all very bad because we go along to the GP with a bit of a sniffle and we expect to get an antibiotic. We have got to break out of this. We have got to stop using them in aquaculture. In fact, it is amazing that 13,600 tons of antibiotics in 2009 were used on animals that were not sick. So, which antibiotic would you like with your bacon, Sir?
In Holland, in 2004, where they have less of a problem with MRSA, they actually do routine screening and, to their amazement, a girl coming in for a routine operation was found to have MRSA – how come? Well, what had happened was she had picked it up from the pigs in her farm. Not only that, this particular variety then spreads, and it spreads very, very quickly throughout the world, and it turns out that 24% of farmers were actually colonised with this strain. So is the meat. Now, it does not matter because it is not poisonous and you are going to cook it, but what is important is you are going to handle it before you cook it, and if you then do this because you have got a little bit of an itch or you have got the onions that are making you cry, you have now colonised your nose with MRSA, which may do you no harm, but if you go into hospital with it and you have a wound and you infect that wound with your MRSA, or you infect other people in the hospital with that MRSA, they have a serious, potentially lethal, problem.
Of course, this could not happen in England, could it? Well, this Christmas 2013, first time ever, MRSA drug resistant staph found in Christmas turkeys in the United Kingdom. And this is really, really important. Just in November, this patient, called BP, was operated on in Vietnam, returns to New Zealand, and he dies of an infection that is totally resistant to everything – there was no antibiotic on Earth could save his life, and he died. So, I want you to imagine the future world, where an infection is so dangerous that even minor symptoms can have you locked in confinement, such as Typhoid Mary, who ended up being locked up in secure hospitals until she died in New York, where you cannot have routine surgery – there would be no prosthetics, not hip replacements, difficult to put lens implants into eyes because the antibiotics are not going to be effective to stop them being infected… Unprotected sex, which is not a wise idea anyway, will become seriously dangerous, like it did in 1915, 1916 and 1917.
It is also important for the plants. Streptomycin resistance destroyed Michigan’s orchards. It is now in Upstate New York this year. If it destroys those orchards, then we are not going to have no apples, no cider – I mean, this is going to be terrible, this world that we are imagining in the future!
There is something that can be done about this, and we do need to think about it, and it Is thinking about this that enabled people to go, “Do you know, if these bacteria are really good at switching just bits of the genome they want to other bacteria, is it not possible that we could make a gene and then use one of those mobile genetic element mechanisms and put it into a human cell, perhaps put it into an embryo, and let the embryo grow up, and an embryo that would otherwise be destined to form an adult who is going to die of, let us say, Huntingdon’s chorea in their twenties, or cystic fibrosis, could live a healthy and normal life.
Well, there are certain problems with that, and one of the problems is that actually doing germ-cell, that is ovary, testes or blastomere genetic engineering is a very, very dangerous thing because it is not just passed onto that person, it is passed onto every one of their generations and, if you get it wrong, and it is very easy to get this wrong because you do not know where the gene is going to be inserted, for a start, you are going to end up with a number of problems. So, the alternative approach is to go and actually try and transfect just certain cells, outside the body, and then put them back in, and then, luckily for us, in the eye, we can actually treat the eye in-situ, and you will know that, last week, some very, very exciting research data came out of Oxford, from Professor MacLaren’s group, which I will discuss at the end.
To transfer genes therapeutically, essentially, we are going to do the same thing. Now, if we have an absent gene, there is a failure of function, which makes it easier because we can just replace this gene with a healthy gene. So, we have got to identify the gene, we have got to make a copy of it, we have got to put it into a mechanism that can get it into the cell, and this could be a vector or could be a naked DNA. Now, human cells are not very good at picking up naked DNA. We could package it into a virus, and viruses are very good at getting into cells. We could use a bacterial plasmid, which gives it some resistance, or we can use nanoparticles, where we wrap it up in a lipid molecule and we get a very big payload of genes in there.
That is not the end of the story. Once we have got it in here, nucleic acids are in a hostile environment. There are lots of these things, called restriction endonucleases, that are present in the cell and their job is to chew up nucleic acids. Their job is to prevent that cell dying from virus infections in the wild, and it cannot tell the difference between an artificial nucleic acid and a viral nucleic acid. So, it is doing its job, but it is a darned nuisance if you want to get this gene through here.
Let us, for argument’s sake, it does, it makes it through this hostile environment, it gets into the nucleus, it incorporates itself into the DNA, and it comes off and then it is translated, and then that DNA goes off and, suddenly, here we have the artificial gene transcribed as a new protein which is the healthy protein, and that disease that is missing that gene is suddenly not a disease anymore. It is cured – as long as that is keeping on being expressed. Now, not all viral vectors allow integration. Some of the genome just is in the cell and it decays very quickly, so you need to repeat the treatment several times. Even the ones that are integrated sometimes silence and they need to be repeated as well.
We get more problems if we have got a variant gene that makes an abnormal protein and the abnormal protein causes the disease – it is a nasty protein instead of a nice protein. Now, that is difficult because, if we just put in a nice protein, we have still got the nasty protein that is still being made, so we have not dealt with that as a problem. But there is a technique coming out here called gene silencing, and we are able, with using small bits of RNA, to specifically bind, using zinc finger and a variety of other technologies such as CRISPR, into this dicer, which is a great big pair of scissors that comes up and chops up the DNA and breaks it down, or, for the other form of silencing, which is miRNAs, it can package it and store it, not necessarily destroy it. This is the gene editing that we are using. We are using these enzymes. Here is the Zinc molecule, here is the Zinc fingers, and they are coming in and, very specifically, they are causing double stranded breaks in DNA. Now, you can chop the DNA to chop out a bad gene. Say it was a mutogenesis you got from, I do not know, smoking cigarettes – you have got a gene defect there. We could identify what that gene is, chop it out, the DNA is then joined up with normal DNA again. The problem is, with an inherited disorder, that does not work because you are going to replace it with the bad gene again, unless you can do something about that gene. But what it is doing is allowing us to target, very precisely, very accurately, certain bits of the DNA. Now, the problem with Zinc fingers are they are a bit too small, so it is quite likely you can find some other bits that are not your gene of choice that you are aiming for, but some other random bits that happen to have the same sequence, and that can happen by chance, frequently enough in a genome that is full of billions of base pairs. So, larger enzymes, such as the CRISPR and the I-Crel homing endonucleases, there is less chance. This can only really be one place in the whole genome that is going to be like this. This technology is advancing. So, we can silence genes by chopping them out, and we can actually home in, make cuts in the DNA to allow us to specifically insert a gene in specific places.
In fact, this is a therapy that is going to be considered for Huntington’s chorea, where these interfering RNAs are going to be introduced, and you can see the bad gene has been snipped out, and we are not going to make the bad protein anymore.
This is the blastomere, which we mentioned before, and this is ex-vivo, which is the more common way of considering, where we put the gene – we take the cells out of the body, we put the gene into them, we culture them, and then we put them back into the body again to replace the missing part. We can do this with non-viral vectors and we can enhance the delivery of these because just a non-viral vector just floating around does not get it, but we can use a gene gun with gold particles, magnetism…there is a bunch of different ways that this can be done, and we can design the polyplexes to be more efficient at being taken up by the cell.
But, most commonly, and what I want to deal with in more detail, are the viral vectors. So, what we do is we load in the new gene into the virus, we sterilise the virus to make sure it is not going to kill you, and then we pop it into the body or into the cell culture, and here it comes, and it corporates, and lo and behold, we have not got the gene here that can start making the good protein.
Adenoviruses are not very good at doing this, and, furthermore, adenoviruses cause upper respiratory tract and common cold problems, so if you are going to inject that into some person, you are very likely to end up with an immune response, and in fact, that did happen in one of the early gene therapy trials and the young patient died. So, adenoviruses, although they have got a big gene load and they are very common, are very difficult to use because of this immune response.
But adeno-associated viruses do not cause disease so much and have a very limited immune response. So, these are now one of the commoner forms of using gene therapy, particularly for eye diseases.
Herpes simplex, you are going to hear a bit more about, because these can be used for neural diseases but also they are very good for cancers such as brain tumours and melanomas because you can put a suicide gene into the vector and then home it into its target and then it is going to kill cancer cells that otherwise could not be killed. So, you are beginning to see, this gene therapy has a little bit more going to it and it’s terribly exciting. The reason we have not seen it widespread and used yet is it is terribly, terribly hard to get it right, get the right vector, make it safe, prove it is safe, get approval and go forward.
And then, finally, retroviruses. Now, we all know what retroviruses are. AIDS is a retrovirus. So, obviously, using retroviruses, you have got to be very careful with them and you have got to remove their infectivity to stop them giving you a rather nasty disease.
Now, this, unfortunately, in 1999, was where young Gelsinger died in the gene therapy trial, just a few days after being injected, from this massive immune response, and that was using the adenoviral vector.
Using adeno-associated virus vectors, we can see there is a number of trials here, in phase one, in phase two, and one in phase three, for treating cancer, haemophilia, Alzheimer’s, arthritis, Parkinson’s, cystic fibrosis. You know, hands up here who does not know somebody or has not come across one of those diseases on this list… But the one I have highlighted is this one, Leber’s, which was the first successful trial, and that is what we are going to talk about when we come onto the eye section.
Now, retroviral vectors, we have mentioned before. If you have this disease, which is the immunodeficiency, you are going to die of infection, so it is very difficult to manage this disease, because you are not making the gene that is necessary for immunity to work. This is “the boy in the bubble” that you will remember, David Vetter, who ended up with terrible psychological problems. He did eventually have a bone marrow transplant but he died of Burkitt’s Lymphoma, transferred to him from his sister’s bone donor, and he died aged twelve.
So, in fact, since there are problems with bone marrow donation, and to have a perfect match for bone marrow donation is extraordinarily difficult, gene therapy is going to be a really exciting way of transferring healthy genes and making healthy products for children with the various different genetic disorders that can cause this.
These lentiviral vectors now are used for Parkinson’s disease and we can see proof of concept, where things are lighting up. It is going, targeted to the right place, seems to be working, and there is some subjective evidence that it is helping.
But what you want to know from me now is, yes, this is all theory, but tell me about something that is really done, that is on the market. Well, here we are…Familial lipoprotein lipase deficiency. This is where you have a lot of fat in your blood, so this can kill you. It can cause pancreatitis and various other things. There is a gene treatment for this, and it is the most expensive drug in the world – it is $1.6 million per treatment.
Amaurosis, which we are coming onto now, is when the eye is not seeing but it looks normal, and this was caused by a bunch of retinal and optic nerve diseases that were not discovered until the ophthalmoscope was discovered, of course, like all these things, an original invention by Charles Babbage, who unfortunately gave it to a Moorfields surgeon who did not do anything with it, so the Germans actually came and invented their own and got on with the process of identifying these retinal diseases.
You will remember that rods and cones, and they have little discs in them, and here is the disc, because then, enlarging the disc, there is a lot of biochemistry going on in this. Furthermore, in the pigment cell that supports it, there is more biochemistry going on that can re-convert this form of Vitamin A back into the form that can be re-loaded into the photoreceptor.
Defects in any of these genes can cause blindness. This is the one that is involved with Leber’s. Others are involved with retinitis pigmentosa, and there is a bunch of different diseases associated with this, and they can be x-linked, they can dominant, they can be autosome or recessive.
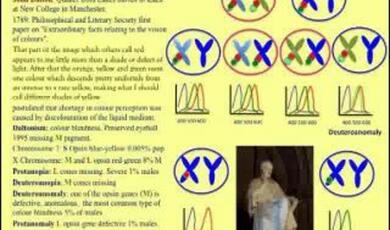
Here is a good x-linked disease, colour blindness. All New World male monkeys are colour-blind and the reason they are colour-blind is that they have only got one X chromosome, and, as we know, males are genetically deficient females. Now, you have the green receptor, for example, here – it is not really green, call it a mid-wave receptor. You have also got a blue receptor elsewhere which are not x-linked. So, you are a deuteronome – you have got two forms of colour receptors that peak, one of them in the mid-range here, for argument’s sake we will call it green. Now, the female, of course, has two X chromosomes, so she is also a deuteronome, two receptors, but it turns out this allele is very polymorphic. That means there are lots of variants in the population, as I told you before – more than one percent. So, some females can have a variant, here, that makes them with three receptors, which gives them the same sort of colour vision as we do as Old World primates. New World primates split off and did not involve the gene duplication on the X that we have. We have this here, on the X chromosome.
They did the gene therapy and they put in a human variant of this gene into the male monkey, here, and lo and behold, they can start to see colours, the reds and so on that they could not see before. And you will think how can you do a colour-test on monkeys? Well, you can. There are various things – he has to push that with his nose if he sees it, and if he does, you give him a drink of juice. It is that simple. It is just like kids, is it not? – they do something good, give them some juice in the bottle and hope Grandma does not see you do it, and eventually you get the right sort of behaviour. It does not take long to potty-train them if you really sort of put your mind to it.
Now, so, this could happen. Interestingly, one of those monkeys was called Dalton, after Dalton who worked out why he might be colour-deficient, incorrectly, as it turns out, but amazing thought that went into what he did.
Leber’s congenital amaurosis, which also occurs in Briard dogs, which are a very old breed that we used for hunting, causes a wipe-out of the retina. This is a dog retina – that is why it does not look like the other pictures. Now, Briards are particularly nice dogs, they are friendly dogs – you see them in films. Chewbacca in Star Wars was actually based on a Briard as well, for those of you who are Star Wars people in the audience, and there always seems to be one or two.
The gene that causes this is that RPE65 protein, the one that recycles Vitamin A into a useful form, and if it does not recycle it, it builds up in the pigment epithelium and it kills the pigment epithelium. If the pigment epithelium dies, then everything above it starts to die, and eventually you end up blind. It takes a while to go completely blind, but you have very poor vision even in childhood.
So, what they did, with two teams, one in the United States and one in the United Kingdom, they injected under the retina – pretty cool surgery, this – the viruses that contain the proper version of RPE65, and lo and behold, they got improvement in vision for some of the patients. It was done as a proof of concept.
Those of you who remember my very first lecture, when I showed the blind patient now, after this treatment, navigating through the maze without bumping into things, now it is not that they are suddenly read War and Peace by candlelight, it is not that sort of success, but what it is is to slow the rate of blindness and to actually, in some patients, improve blindness, and also to show it is safe, which they did.
Retinitis pigmentosa is another great disease to target because the commonest form of this is X-linked – i.e. a protein is missing. It is boys, and they are going to come and recruit because they do not have that danger signal that women do, so they are going to say, “Yes, sure, inject me – whatever it takes, I want a bit of it!” That is the norm and this is of retinitis pigmentosa. So, there are a number of treatments, gene treatments, that may alter the progress of this disease.
Then, finally, I want to mention choroideremia, which is where the big trial has just come out of Oxford, again showing positive effects in this otherwise relentlessly progressive disease.
Now, can we use this for other treatments? Yes, we can. We can use it for things like macular degeneration. Instead of having an injection every month now, we can give you a viral transfer, potentially, and that makes the anti-VEGF for you. There are some problems with that. God made VEGF for a reason. Now, I know, when it goes wrong, you get macular degeneration, but it does other things as well, and we do not really fully understand this to permanently give someone an anti-VEGF virus that could spread out of the system, go somewhere else and cause some terrible problem. So, these trials are underway, and the good thing about the eye though, as opposed to other organs, we can limit it to the eye, so it is less likely to spread out and cause damage elsewhere.
I just, very finally, wanted to just keep you alert to some emerging treatments. Optogenetics is one. If you have no retinal receptors left, such as in retinised pigmentosa, you can infect with virus an artificial light receptor, which means the cells above can now react to light and get the pathway working again.
We can use stem-cells. We have got artificial vision – I have just come back from the Artificial Vision Conference, where various different types were discussed, both the American, which is this, which has restored some useful vision in patients, and one patient can actually draw letters, from complete blindness. I mean, this is miraculous!
Finally, you can get an inkjet to make a retina. In Cambridge, Keith Martin, who is the Professor there, he got a Piezoelectric printer, put some cells into it, and was able to print out a retinal mosaic, with ganglion cells and some structural cells for it. This is extraordinary.
Ladies and Gentlemen, I hope that you found that interesting and stimulating and that it will help you understand some of the newspaper articles that you are going see, or you are going to be bombarded with, over the next few years.
I would like to thank my patients, as always, and the colleagues and staff at the Hospital – without them, I could not have done any of this work, of course the Gresham College staff and the Museum of London AV, which was fantastic today – thank you very much indeed. If you have any burning questions or things, you can contact through the College.
© Professor William Ayliffe, 2014


 Login
Login